Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 and Eu
and Eu ions onto sedimentary rock in the presence of gamma-irradiated humic acid
ions onto sedimentary rock in the presence of gamma-irradiated humic acidZhao, Q.*; Saito, Takeshi*; Miyakawa, Kazuya; Sasamoto, Hiroshi; Kobayashi, Taishi*; Sasaki, Takayuki*
Journal of Hazardous Materials, 428, p.128211_1 - 128211_10, 2022/04
Times Cited Count:5 Percentile:59.24(Engineering, Environmental)The influence of humic acid and its radiological degradation on the sorption of Cs and Eu
and Eu by sedimentary rock was investigated to understand the sorption process of metal ions and humic substances. Aldrich humic acid (HA) solution was irradiated with different doses of gamma irradiation using a Co-60 gamma-ray source prior to the contact between the metal ions and the solid sorbent. The HA molecule decomposed to smaller molecules with a lower complexation affinity. Batch sorption experiments were performed to evaluate the effect of gamma-irradiated HA on the sorption of Cs
by sedimentary rock was investigated to understand the sorption process of metal ions and humic substances. Aldrich humic acid (HA) solution was irradiated with different doses of gamma irradiation using a Co-60 gamma-ray source prior to the contact between the metal ions and the solid sorbent. The HA molecule decomposed to smaller molecules with a lower complexation affinity. Batch sorption experiments were performed to evaluate the effect of gamma-irradiated HA on the sorption of Cs and Eu
and Eu ions. The addition of non-irradiated HA weakened the sorption of Eu because of the lower sorption of the neutral or negatively charged Eu-HA complexes compared with free Eu ions. The sorption of monovalent Cs ions was barely affected by the presence of HA and its gamma irradiation. The concentration ratio of HA complexed species and non-complexed species in the solid and liquid phases was evaluated by sequential filtration and chemical equilibrium calculations. The ratios supported the minimal contribution of HA to Cs sorption. However, the concentration ratio for Eu
ions. The addition of non-irradiated HA weakened the sorption of Eu because of the lower sorption of the neutral or negatively charged Eu-HA complexes compared with free Eu ions. The sorption of monovalent Cs ions was barely affected by the presence of HA and its gamma irradiation. The concentration ratio of HA complexed species and non-complexed species in the solid and liquid phases was evaluated by sequential filtration and chemical equilibrium calculations. The ratios supported the minimal contribution of HA to Cs sorption. However, the concentration ratio for Eu in the liquid phase was high, indicating that the complexing ability of HA to Eu
in the liquid phase was high, indicating that the complexing ability of HA to Eu was higher than that of HA to Cs
was higher than that of HA to Cs ions. Therefore, the sorption of free Eu
ions. Therefore, the sorption of free Eu would predominate with the gamma irradiation dose applied to the HA solution under a radiation field near the HLW package.
would predominate with the gamma irradiation dose applied to the HA solution under a radiation field near the HLW package.
Yamaguchi, Tetsuji; Ohira, Saki; Hemmi, Ko; Barr, L.; Shimada, Asako; Maeda, Toshikatsu; Iida, Yoshihisa
Radiochimica Acta, 108(11), p.873 - 877, 2020/11
Times Cited Count:7 Percentile:65.65(Chemistry, Inorganic & Nuclear)Tachi, Yukio; Suyama, Tadahiro*; Mihara, Morihiro
JAEA-Data/Code 2019-021, 101 Pages, 2020/03
Sorption of radionuclides in cement and bentonite as engineered barrier materials, and rocks as natural barrier is the one of key processes in the performance assessment of geological disposal of TRU and high-level waste. The magnitude of sorption, expressed normally by a distribution coefficient (K ), needs to be measured and determined taking into account the properties of barrier materials and geochemical conditions and associated uncertainty in the performance assessment. The basic concept for TRU waste disposal contains cementitious materials as an engineered barrier materials, in addition to bentonite and rock. It is therefore needed to consider the effects of the cement degradation and co-existing substances such as nitrates on radionuclide sorption. This report focused on data acquisition of distribution coefficient (K
), needs to be measured and determined taking into account the properties of barrier materials and geochemical conditions and associated uncertainty in the performance assessment. The basic concept for TRU waste disposal contains cementitious materials as an engineered barrier materials, in addition to bentonite and rock. It is therefore needed to consider the effects of the cement degradation and co-existing substances such as nitrates on radionuclide sorption. This report focused on data acquisition of distribution coefficient (K ) by batch sorption experiments for the systems coupling barrier material-chemical condition-radionuclides that are needed to consider for the performance assessment of geological disposal of TRU waste. The barrier materials considered are ordinary Portland cement (OPC), degraded OPC and tuff rock. The chemical conditions are distilled water and synthetic seawater equilibrated with OPC and those containing nitrates and ammonium salts, etc. The radionuclides considered are organic carbon, inorganic carbon, Cl, I, Cs, Ni, Se, Sr, Sn, Nb, Am and Th. Although K
) by batch sorption experiments for the systems coupling barrier material-chemical condition-radionuclides that are needed to consider for the performance assessment of geological disposal of TRU waste. The barrier materials considered are ordinary Portland cement (OPC), degraded OPC and tuff rock. The chemical conditions are distilled water and synthetic seawater equilibrated with OPC and those containing nitrates and ammonium salts, etc. The radionuclides considered are organic carbon, inorganic carbon, Cl, I, Cs, Ni, Se, Sr, Sn, Nb, Am and Th. Although K values have been partly reported previously as RAMDA (Radionuclide Migration Datasets) for the performance assessment in the TRU-2 report, these results and addition K
values have been partly reported previously as RAMDA (Radionuclide Migration Datasets) for the performance assessment in the TRU-2 report, these results and addition K data are reported with the details of experimental methods and conditions.
data are reported with the details of experimental methods and conditions.
 as a source of atmospheric I
as a source of atmospheric I
Watanabe, Kosuke*; Matsuda, Shohei; Cuevas, C. A.*; Saiz-Lopez, A.*; Yabushita, Akihiro*; Nakano, Yukio*
ACS Earth and Space Chemistry (Internet), 3(4), p.669 - 679, 2019/04
Times Cited Count:8 Percentile:42.7(Chemistry, Multidisciplinary)The photooxidation of aqueous iodide ions (I
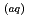 ) at sea surface results in the emission of gaseous iodine molecules (I
) at sea surface results in the emission of gaseous iodine molecules (I
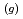 ) into the atmosphere. It plays a certain role in the transport of iodine from ocean to the atmosphere in the natural cycle of iodine. In this study, we determined the photooxidation parameters, the molar absorption coefficient (
) into the atmosphere. It plays a certain role in the transport of iodine from ocean to the atmosphere in the natural cycle of iodine. In this study, we determined the photooxidation parameters, the molar absorption coefficient (
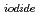 (
( )) and the photooxidative quantum yields (
)) and the photooxidative quantum yields (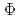
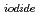 (
( )) of I
)) of I
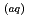 , in the range of 290-500 nm. Through the investigation of the influence of pH and dissolved oxygen (DO) on
, in the range of 290-500 nm. Through the investigation of the influence of pH and dissolved oxygen (DO) on 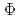
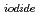 (
( ), the subsequent emission rates of I
), the subsequent emission rates of I
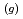 following the photooxidation of I
following the photooxidation of I
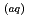 in deionized water solution (pH 5.6, DO 7.8 mg L
in deionized water solution (pH 5.6, DO 7.8 mg L ) and artificial seawater solution (pH 8.0, DO 7.0 mg L
) and artificial seawater solution (pH 8.0, DO 7.0 mg L ) were estimated. A global chemistry-climate model employed herein to assess the I
) were estimated. A global chemistry-climate model employed herein to assess the I
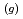 ocean emission on a global scale indicated that the photooxidation of I
ocean emission on a global scale indicated that the photooxidation of I
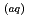 by solar light can enhance the atmospheric iodine budget by up to
by solar light can enhance the atmospheric iodine budget by up to  8% over some oceanic regions.
8% over some oceanic regions.
Iida, Yoshihisa; Yamaguchi, Tetsuji; Tanaka, Tadao; Hemmi, Ko
Journal of Nuclear Science and Technology, 53(10), p.1573 - 1584, 2016/10
Times Cited Count:10 Percentile:67.99(Nuclear Science & Technology)The sorption behavior of thorium (Th) onto granitic rock and its major constituent were investigated by batch sorption experiments. Experiments were carried out under variable pH and carbonate concentrations. Distribution coefficients decreased with increased carbonate concentrations and showed the minimal value at pH 9-10. This sorption tendency was likely due to forming the hydroxide-carbonate complexes of Th in the solutions. The order of sorbability for Th was mica  feldspar
feldspar  quartz = granite. The sorption behaviors of Th onto these minerals were analyzed by the triple-layer surface complexation model with the Visual Minteq computer program. The model calculations assuming the inner-sphere surface complexation of Th were able to explain the experimental results reasonably well. It was shown that the sorption behavior of Th onto granite can be explained primarily by the complexation with the surface sites of feldspar.
quartz = granite. The sorption behaviors of Th onto these minerals were analyzed by the triple-layer surface complexation model with the Visual Minteq computer program. The model calculations assuming the inner-sphere surface complexation of Th were able to explain the experimental results reasonably well. It was shown that the sorption behavior of Th onto granite can be explained primarily by the complexation with the surface sites of feldspar.
Mukai, Masayuki; Ueda, Masato; Inada, Daisuke; Yukawa, Kazuhiko; Maeda, Toshikatsu; Iida, Yoshihisa
Proceedings of International Symposium NUCEF 2005, p.219 - 224, 2005/08
For better quantitative understanding of radionuclide migration for safety assessment of geologic disposal, JAERI has been conducting experimental and modeling studies on influences of humic substances, highly alkaline conditions and colloids on sorptive and diffusional behavior of TRU in geologic materials. In the absence of fulvic acid, one of humic substances, diffusion of Am through a tuff sample was not detected. By adding fulvic acid, Am was detected in the downstream cell, which indicates the diffusion through the sample. Highly alkaline conditions arisen from cementitious materials may spread by altering chemical and physical properties of geologic materials. Through-diffusion experiments of alkaline species in granite showed that the effective diffusion coefficient of Ca and OH
and OH in a cement-equilibrated aqueous solution were found to be higher by almost two orders of magnitude than Na
in a cement-equilibrated aqueous solution were found to be higher by almost two orders of magnitude than Na and OH
and OH in a NaOH solution. Radionuclide migration can be enhanced by colloids, and thus a calculation code describing the effect of colloids on radionuclide migration has been required.
in a NaOH solution. Radionuclide migration can be enhanced by colloids, and thus a calculation code describing the effect of colloids on radionuclide migration has been required.
Yamaguchi, Tetsuji; Nakayama, Shinichi; Yoshida, Takahiro
Radiochimica Acta, 92(9-11), p.677 - 682, 2004/12
Times Cited Count:6 Percentile:40.63(Chemistry, Inorganic & Nuclear)Adsorption of actinides onto negatively charged mineral surfaces was investigated under conditions that actinides were predominantly present as anionic complex species: Th(CO )
)
 , Am(CO
, Am(CO )
)
 , Np(CO
, Np(CO )
) (OH)
(OH)
 , UO
, UO (OH)
(OH)
 , NpO
, NpO (OH)
(OH)
 , Sn(OH)
, Sn(OH)
 and Pb(OH)
and Pb(OH)
 . These solutions were left to stand for 2 days to confirm these elements stable in dissolved state, and then contacted with minerals,
. These solutions were left to stand for 2 days to confirm these elements stable in dissolved state, and then contacted with minerals,  -Al
-Al O
O or SiO
or SiO (AEROSIL, specific surface area: 10
(AEROSIL, specific surface area: 10 m
m kg
kg ). After desired contact time for 2 days or more, the solutions were ultra-filtered through 10
). After desired contact time for 2 days or more, the solutions were ultra-filtered through 10 -molecular-weight cutoff Millipore filters and the concentrations of the elements in the filtrates were determined. The sorption experiments were performed at room temperature (25
-molecular-weight cutoff Millipore filters and the concentrations of the elements in the filtrates were determined. The sorption experiments were performed at room temperature (25 C) under Ar. Distribution coefficients decreased with the increasing pH and with increasing carbonate concentrations. The monotonous decrease in the distribution coefficients in the investigated pH range suggests that the electrostatic repulsion was a dominant interaction between anionic complex species of actinides and negatively charged mineral surfaces.
C) under Ar. Distribution coefficients decreased with the increasing pH and with increasing carbonate concentrations. The monotonous decrease in the distribution coefficients in the investigated pH range suggests that the electrostatic repulsion was a dominant interaction between anionic complex species of actinides and negatively charged mineral surfaces.
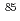 Sr,
Sr,  Np,
Np,  Pu and
Pu and  Am in loess media (Joint research)
Am in loess media (Joint research)Tanaka, Tadao; Mukai, Masayuki; Maeda, Toshikatsu; Matsumoto, Junko; Ogawa, Hiromichi; Li, S.*; Wang, Z.*; Wang, J.*; Guo, Z.*; Zhao, Y.*
JAERI-Research 2002-034, 20 Pages, 2002/12
Adsorption mechanisms and models of 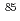 Sr(II),
Sr(II),  Np(V),
Np(V),  Pu(IV) and
Pu(IV) and  Am(III) on the loess were investigated from their adsorption and desorption properties. The distribution coefficient of
Am(III) on the loess were investigated from their adsorption and desorption properties. The distribution coefficient of 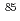 Sr and
Sr and  Np was 2 - 3 orders of magnitude smaller than that of
Np was 2 - 3 orders of magnitude smaller than that of  Pu and
Pu and  Am. The adsorption of
Am. The adsorption of 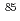 Sr and
Sr and  Np was mainly controlled by the ion exchange reaction. On the other hand, the adsorption of
Np was mainly controlled by the ion exchange reaction. On the other hand, the adsorption of  Pu and
Pu and  Am was mostly controlled by the selective chemical reactions with Fe and Mn oxyhydroxide/oxide and humic substances. On the basis of the experimental results, several types of adsorption models of the radionuclides, considering elemental concentrations, adsorption mechanisms and kinetics, were proposed for setting up the analytical systems of radionuclide migration in the loess media.
Am was mostly controlled by the selective chemical reactions with Fe and Mn oxyhydroxide/oxide and humic substances. On the basis of the experimental results, several types of adsorption models of the radionuclides, considering elemental concentrations, adsorption mechanisms and kinetics, were proposed for setting up the analytical systems of radionuclide migration in the loess media.
Kozai, Naofumi; Inada, Koichi*; Kozaki, Tamotsu*; Sato, Seichi*; Ohashi, Hiroshi*; Bamba, Tsunetaka
Journal of Contaminant Hydrology, 47(2-4), p.149 - 158, 2001/02
Times Cited Count:15 Percentile:41.9(Environmental Sciences)no abstracts in English
; Usuda, Shigekazu
Journal of Alloys and Compounds, 271-273, p.244 - 247, 1998/00
Times Cited Count:17 Percentile:69.24(Chemistry, Physical)no abstracts in English
; ;
Nihon Genshiryoku Gakkai-Shi, 25(8), p.658 - 663, 1983/00
Times Cited Count:0 Percentile:0.02(Nuclear Science & Technology)no abstracts in English
Nuclear Instruments and Methods, 114(1), p.65 - 69, 1974/01
no abstracts in English